- Chemistry
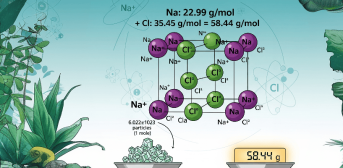
In chemistry contexts, “mass of NaCl (Sodium chloride)” almost always refers to its molar mass. Sodium chloride (NaCl), commonly known as “table salt,” is so ubiquitous we rarely pause to consider its fundamental nature. Its humble grains embody a sophisticated dance of electrons and ions — one that has shaped “chemistry, biology, and industry” for centuries.