Mass of NaCl
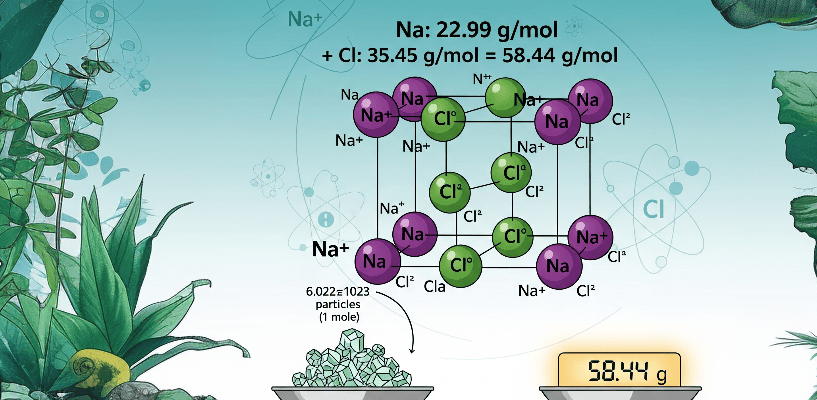
Sodium chloride (NaCl) is an ionic compound. Its “crystal lattice” is built from positively charged sodium ions (Na+) and negatively charged chlorine ions (Cl—), held together by strong electrostatic forces.
To find the mass of “one mole” of NaCl, we need the combined mass of one mole of “sodium atoms” and one mole of “chlorine atoms.” In practice, “molar masses” of a compound/molecule are derived from neutral “atomic masses,” and “ionic masses” are not commonly tabulated separately.
Molar Mass of NaCl = Molar Mass of Na + Molar Mass of Cl
= Atomic Mass of Na + Atomic Mass of Cl
= 22.99 g/mol + 35.45 g/mol,
Thus, Molar Mass of NaCl = 58.44 g/mol,
58.44 g/mol is the mass of 6.022 x 1023 “molecules” of NaCl,
58.44 g/mol is the mass of 6.022 x 1023 “formula units” of NaCl.
Note: – The smallest repeating unit (in a crystal lattice) is often called a “formula unit,” not a molecule.
If you need the “mass of a single NaCl unit” (i.e., mass of a molecule of NaCl), divide “58.44 g/mol” by Avogadro’s number:
“58.44 g/mol ÷ 6.022 x 1023 molecules/mol ≈ 9.71×10−23 g/molecule”
What “Mass” Means in Chemistry? -
In everyday language, “mass” and “weight” can blur together — but in chemistry, mass has a precise meaning tied to the “number of particles” involved. In chemistry, “mass” takes on a ‘precise, measurable’ meaning, especially when we talk about the “molar mass.” This isn’t about weight in a spiritual sense, but the mass of a “specific quantity” of substance (i.e., mass of one mole of substance).
Mass is the “amount of matter” in an object, while weight is the force exerted on that mass by gravity. “Molar mass” refers to the mass.
Chemists define the “molar mass” as the mass of one mole (6.022 × 1023 entities) of a substance, expressed in “grams per mole” (g/mol). Think of a mole as a chemist’s “dozen,” but instead of 12, it’s a colossal 6.022 x 1023 (Avogadro’s number). That’s 602,200,000,000,000,000,000,000 particles!
Why Do We Use Molar Mass (Not Single-Unit Mass)? -
A single NaCl “molecule” (ion pair) has a mass on the order of 10—23 g (far too small to weigh directly). Analytical balances in labs measure grams (10—3 g) at best, so working with moles (1023 units) brings masses into a measurable range.
Chemical equations are balanced in “moles,” not individual molecules. Using “molar masses” lets us calculate exactly how many grams of each reactant are needed (and how many grams of product will form). Example: To make 0.5 mol of NaCl, weigh out
“0.5 mol × 58.44 g/mol = 29.22 g of salt.”
Every compound’s molar mass (g/mol) follows the same rule — sum of atomic masses — so it’s a universal “translator” between microscopic counts and macroscopic weights. Properties like “boiling‑point elevation or osmotic pressure” depend on the number of dissolved particles (moles), not their individual mass.
Why Avogadro’s Number? -
Avogadro’s number isn’t arbitrary — it was chosen specifically to link the “atomic scale” with the “gram scale.” It serves as the conversion factor between:
- Atomic mass units (u) (also called Dalton, Da) → used to measure mass at the atomic level.
- Grams (g) → used to measure mass in the lab.
Avogadro’s number keeps “molar mass in g/mol” numerically equal to “atomic/formula mass in u,” which simplifies all chemical calculations. It means “1 mole of any substance has a mass in grams” numerically equal to its “atomic/formula mass in u.”
Example: NaCl has a formula mass of “58.44 u” → Its molar mass is “58.44 g/mol.” No unit conversions needed!
Why Does “Mass” Matters? -
Making saline solution (like for contacts or IV drips) requires precise concentrations (e.g., 0.9% w/v NaCl). “Molar mass” is essential for calculating how much salt to dissolve in water to achieve a specific molarity (moles per liter).
Food labels list sodium content. Knowing that 39.4% of NaCl’s mass is sodium (“23 g Na / 58.44 g NaCl” ≈ 0.394 or 39.4%) helps us understand “how much ‘actual salt’ that sodium content represents.” If a can of soup has 600 mg sodium per serving, then it can be thought as “1.53 g of salt” (600 mg/0.394 ≈1525 mg NaCl).
Knowing the molar mass allows us to convert between “the mass of a substance we can weigh” and “the number of moles,” which tells us how many particles (and thus how much reactivity) we have. Need 0.5 moles of salt for a reaction? That’s “0.5 mol * 58.44 g/mol = 29.22 grams.”
That’s all friends.
Suggestions or corrections for this page can be submitted from the “contact us” page.
Ads Section

